Temporary Anion States and Dissociative Electron Attachment

A complete picture of the electronic structure requires the knowledge of both the filled and the empty levels, equally important from the thoretical and the reactivity points of view. However, while ionization energy data supplied by Photoelectron Spectroscopy (PES) are easily available in the literature, the complementary electron affinity data are still sparse.
Electron Transmission Spectroscopy (ETS) is one of the most suitable means for studying the virtual orbital structure of gas-phase molecular systems. This electron-molecule scattering technique takes advantage of the sharp variations in the electron-molecule scattering cross section associated with temporary electron capture into vacant orbitals. These processes of temporary anion formation are referred to as shape resonances, and the energies at which they occur (vertical attachment energies, VAEs) are the negative of the vertical electron affinities. Our ETS apparatus [1] is in the format devised by Sance and Schulz [2]. To enhance the visibility of the sharp resonance structures, the electron impact energy is modulated with a small ac voltage and the derivative of the electron current transmitted through the gas sample is measured by a synchronous lock-in amplifier. The energy resolution of the incident electron beam is about 40 meV with a beam intensity of 5x10-9 A. ETS is complementary to the more diffuse PES, which measures ionisation energy values and supplies the valence filled orbital structure in gas-phase samples. The two techniques together can supply a complete picture of the valence electronic structure.
When the anion energy is above the threshold for dissociation, the electron attachment process (resonance) may follow a dissociative channel (in kinetic competition with simple detachment of the extra-electron). In this case, a long-lived negative fragment and a neutral radical are formed. Dissociative Electron Attachment Spectroscopy (DEAS) measures the yield of negative fragments (selected with a mass filter), as a function of the electron impact energy.
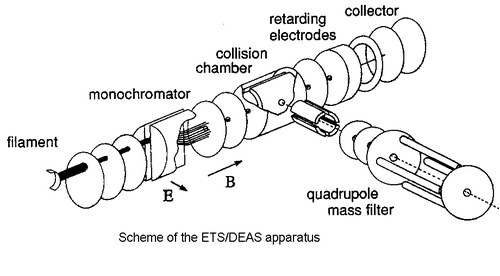
The collision chamber of our ETS apparatus has been modified in order to allow for ion extraction (through a 3 mm diameter hole) at 90ø degrees with respect to the electron beam direction [3]. Ions are then accelerated and focussed toward the entrance of a quadrupole mass filter. Alternatively, a more intense (but not mass discriminated) signal related to the total ion current incident on the collision chamber (0.8 cm far from the electron beam) can be recorded.
The experimental data are interpreted with the aid of proper theoretical calculations. A theoretical approach adequate for describing the energetics of temporary anion formation involves difficulties not encountered for the cation states. The conceptually most correct approach is the calculation of the total electron scattering cross section. Alternatively, bound-state calculations can be used, but in conjunction with stabilisation methods, in order to account for the fact that during a resonance process the extra electron is confined to the molecule. However, it has been shown that the neutral state virtual orbital energies obtained with HF or B3LYP calculations supply a good linear correlation with the corresponding experimental vertical attachment energies.
Building of the ETS/DEAS apparatus in Bologna followed a stage at the Laboratory of Prof. P.D. Burrow, Department of Physics and Astronomy, University of Lincoln-Nebraska. Our ETS/DEAS apparatus is one of the few existing in the world, and favoured scientific collaborations with researchers of foreign Universities: P.D. Burrow (University of Nebraska, Lincoln,m USA), K.D. Jordan (University of Pittsburg, USA), H.D. Martin (University of Dusseldorf), M. Tronc (University Pierre et Marie Curie, Paris VI), A.P. Hitchcock (University McMaster, Hamilton, Canada), J. Tamariz (University of Mexico City), L. Szepes e L. Nyulaszi (University of Budapest), J.P. Schermann (University of Paris 13, Villetaneuse), J. Nixon (University of Sussex, Brighton), N.L. Asfandiarov and S.A. Pshenichnyuk (Academy of Sciences, Ufa, Russia).
The research activity is devoted to the study of electronic structures, orbital interactions and nature of chemical bonds in gas-phase molecular systems. A multidisciplinary approach, including all the above mentioned techniques and theoretical calculations, was employed to characterize the filled and empty level structures of organic monomers and oligomers, extrapolating the results to the corresponding conducting polymers. In particular, we used DEAS to determine the efficiency of intramolecular electron transfer between non-bonded functional groups. These delicate measurements are not only concerned with the energy of the maxima observed in the negative ion currents and the nature of the negative fragments produced, but also with the quantitative aspects, i.e., determination of the absolute cross-sections. ETS and DEAS were employed to characterize (in energy and localization properties) temporary anion states of organic and organometallic compounds (a survey is given in Ref. [4]), following several lines of research:
- systematic investigation on the acceptor properties of hydrocarbons containing elements of the main groups (14-17);
- metal-ligand interactions and charge distributions in transitional complexes;
- frontier electronic structure in compounds of environmental and biochemical interest.
[1] A. Modelli, D. Jones and G. Distefano, Chem. Phys. Lett., 86 (1982) 434.
[2] L. Sanche and G.J. Schulz, J. Phys. Rev. A, 5 (1972) 1672.
[3] A. Modelli, A. Foffani, F. Scagnolari and D. Jones, Chem.Phys. Lett., 163 (1989) 269.
[4] A. Modelli, Trends in Chemical Physics (Research Trends) , 6 (1997) 57-95