Aerobic biodegradation of materials in soil
The widespread and ever growing use of plastics as a convenient alternative to a variety of other materials is based on the successful formulation of a wide range of polymer materials with mechanical and physical properties tunable to the requirements of the specific application. However, because of the increasing attention being paid to environmental problems, in many instances polymer resistance to biological degradation is regarded as a limit to be overcome, especially in plastics applications where a restricted service-time is required and disposal after use is a concern. Hence the search for materials that associate suitable properties with eco-compatibility. Because of their low cost, biodegradability and high specific mechanical properties, natural vegetable fibers are good candidates as reinforcing agents in polymer composites.
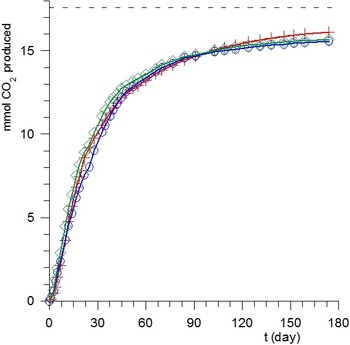
In this laboratory the standard ASTM D 5988-96 method (which measures the evolution of CO2 as a function of time) has been implemented to determine the rate and extent of biodegradation of various materials in the soil in aerobic conditions. In spite of the complexity of the system, the kinetics of degradation of powders of starch, PHB and PCL [1], and flax fibers [2] are closely reproduced by simple first order kinetics for solution reactions.
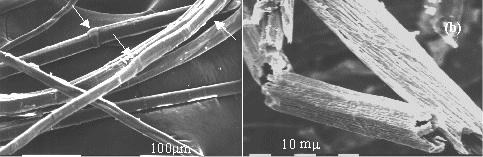
The mechanical properties of fiber-reinforced polymer composites depend on the degree of adhesion between matrix and fibers. As reinforcement is constituted of surface modified vegetable fibers, it is important to verify that after chemical modification the fibers maintain a sufficient degree of biodegradability. Our results demonstrated [2] that the chemical suitable modifications of flax fibers do not significantly affect their total biodegradablity. SEM micrographs of flax fibers before biodegradation and after 13 days of exposure clearly show the effects of biodegradation.
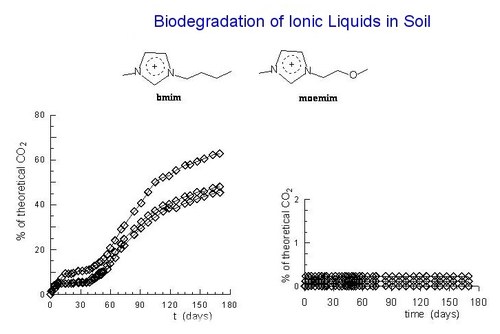
Aerobic biodegradation of ionic liquids in soil was monitored for the first time. The tests, followed over six months according to ASTM D 5988-96, were carried out on the four ionic liquids obtained from 1-R-3-methylimidazolium cations, with R = CH3(CH2)3 and CH3O(CH2)2, and the tetrafluoroborate and dicyanamide counter anions. The n-butyl derivatives, after an induction period of about two months, were found to be degradable, although the degradation rate with the dicyanamide anion was smaller. In contrast, no significant production of CO2 was observed in the tests with the methoxyethyl derivatives.
Calculations at the B3LYP/6-31G(d) level were carried out to characterize the atomic charge distributions and frontier orbital structures of 1-alkyl-3-methylimidazolium cations and point out the changes caused by replacement of a CH2 group of the alkyl chain with an oxygen atom. The calculations predict an overall negative charge on the nitrogen atoms of the imidazolium-based cations. The energies of the highest occupied pi MO and lowest empty pi* MO are only slightly perturbed by the length and nature of the alkyl chain. However, the electron-donor properties of the oxy derivatives are radically increased. The HOMO becomes a lone pair orbital mainly localized on the oxygen atom, and its ionization energy is sizeably smaller than that of the outermost ring pi MO.
1) A. Modelli, B. Calcagno and M. Scandola, J. Environ.Polym. Degrad., 7 (1999) 109 : "Kinetics of aerobic polymer degradation in soil by means of the ASTM D 5988-96 standard method".
2) A. Modelli, G. Rondinelli, M. Scandola, J. Mergaert and M.C. Cnockaert, Biomacromolecules, 5 (2004) 596 : "Biodegradation of chemically modified flax fibers in soil and 'in vitro' with selected bacteria".
3) A. Modelli. A. Sali, P. Galletti and C. Samorì, Chemosphere, 73 (2008) 1322-1327: “Biodegradation of oxygenated and non-oxygenated imidazolium based ionic liquids in soil”.